Is Fireworks Exploding Endothermic or Exothermic
In exothermic changes energy is released and in endothermic changes energy is absorbed. This reaction is very fast and exothermic which means it gives off energy as heatand anytime you have a very fast and hot reaction you get an explosion.

Key Question What Is The Difference Between Exothermic And Endothermic Reactions Warm Up An Increase In Temperature Makes A Reaction Speed Ppt Download
Burning wood fireworks and alkali metals added to water.

. This launches the fireworks into the sky and the heat from this explosion is what provides the energy to create the colors. _____ Chemical bonds form when heat is released. Exploding fireworks is chemical change chemical reaction because new substances are formed the atoms are rearranged and the reaction is followed by an energy change energy is released.
No it is exothermic. An instant ice pack turning cold and ice melting into water. An explosion is an oxidation reaction that creates large amounts of hot gases in.
Most spontaneous chemical reactions are exothermic - they release heat and warm up their surroundings. Because energy in the form of sunlight is required to be put into the system to weaken the strong C-C bonds of a plastic. An exothermic reaction gives off heat.
On the other hand an exploding firecracker and you dont see many of them around these days alas my lost youth is an. K - University grade. Relying on what chemicals and or circumstances are inside a certain method it will likely be either endothermic or exothermic.
2 Show answers Another question on Chemistry. Physical change Biology Need help with these two. The fuel oxidizes burns quickly causing a great buildup in pressure that eventually leads to solids and gases bursting across the sky in colorful patterns.
Is photosynthesis an exothermic or endothermic reaction. _____ Classify the following descriptions as endothermic absorbs energy or exothermic releases energy. The explosion of fireworks is an exothermic redox reaction.
A clue that it is a chemical change is that energy was given off. To buffer her reaction she. The explosion of fireworks is an exothermic redox reaction.
Are fireworks exploding a physical or chemical change. Write ENDO for endothermic and EXO for exothermic on the blank next to each description. Because the firework when it exploded released energy in the form of light.
This reaction is spontaneous. This is like the 4th time Im posting this. Spontaneous Endothermic Reactions.
I remember these as follows. Explosions release heat and light because the energy of the products of those reactions are less. Fireworks exploding and gasoline burning Endothermic.
Fireworks exploding in the sky and giving off light are examples of endothermic or exothermic change Answer It is Exothermic Change becauseExothermic is a chemical reaction that releases energy it could be light or heat. In an exothermic reaction heat is. This reaction is very fast and exothermic which means it gives off energy as heatand anytime you have a very fast and hot reaction you get an explosion.
Fireworks exploding in the sky and giving off light are examples of endothermic or exothermic change was asked on May 31 2017. This launches the fireworks into the. When fireworks explode heat is released.
As anyone who has ever made a film canister rocket knows good rocket design is key. Endothermic vs Exothermic Reaction DRAFT. Breaking of bonds is always endothermic.
Is this exothermic or endothermic and why. As the amount of gas in a sealed container increases so does the pressure in the container. This is a spontaneous exothermic nuclear reaction.
Choose the answer that best describes what is happening. Why dont they just explode on the ground where theyre lit. You mix the pks of succinic acid are 421 and 564.
Consider the hydrolysis of an ester. Exploding fireworks paper burning and cellular respiration such as humans breathing all are examples of exothermic reactions in which heat is released by the chemical reaction. An exothermic reaction is not enough to generate the heavens-bound explosion that Western explorers and tradesmen brought from China to Italy during the Renaissance.
Formation of bonds is always exothermic. Fireworks exploding in the sky and giving off light are an example of an 1 point exothermic change. Using Gibbs Free energy equation - is the Ä G for thereaction of fireworks exploding positive or negative.
The fuel oxidizes burns quickly causing a great buildup in pressure that eventually leads to solids and gases bursting across the sky in colorful patterns. Fireworks explode above your head. It depends on the particular reaction.
Two points to remember. - This wouldnt be a physical change but instead a chemical change. An endothermic reaction uses heat from the surroundings to make it go.
How many gramsa graduate student at sdsu wants to measure the activity of a particular enzyme at ph 40. RCOOR_1 HOH RCOOH R_1OH We could consider this simplistically as the breaking of two old bonds and the formation of two new bonds. There are two types of reaction.
Fireworks have very fast chemical reactions that are and highly exothermic. When a radioactive atom splits up it releases energy. Endothermic reaction chemical reaction that absorbs more energy than it releases and exothermic.
A glow stick glowing and a heat pack becoming warm Exothermic. It is an exothermic reaction because energy is being released in the form of heat. Ä G is Negative because this is an exothermic reaction and there is a decrease in entropy.
A piece of plastic that becomes brittle after exposure to sunlight has undergone an endothermic reaction.

Endothermic Exothermic Chemical Reactions Classifying Reactions Reactions Can Be Classified Based On Whether The Change Involves An Absorption Of Ppt Download

Endothermic Exothermic Chemical Reactions Classifying Reactions Reactions Can Be Classified Based On Whether The Change Involves An Absorption Of Ppt Download

Understanding Endothermic And Exothermic Reactions Chemistry Experiments Exothermic Reaction Chemical Reactions
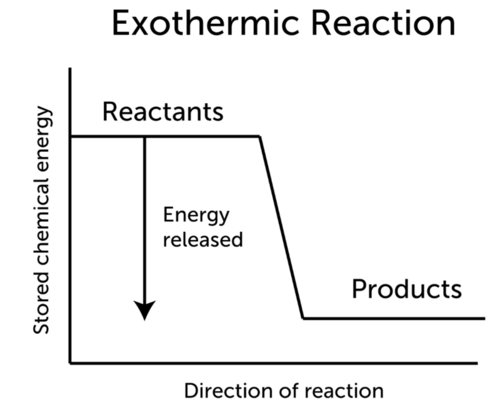
Exothermic Reaction Ck 12 Foundation

5 1 Exothermic And Endothermic Reactions 18 October 2015 Exoskeletons Endoskeletons What Do The Words Endo And Exo Mean Before A Word Ppt Download

Changes In Matter Chapter 2 2 Continued Ppt Notes Include Drawing In Science Journal Ppt Download
Why Do Exothermic Reactions Occur Spontaneously How Do You Explain This Phenomenon Quora
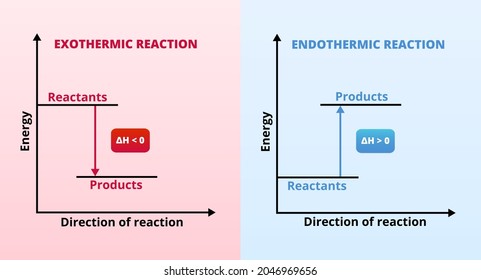
Exothermic Reaction Images Stock Photos Vectors Shutterstock

Chemical Energy Student Notes I Ppt Download

Introduction To Chemical Reactions Flashcards Quizlet

Chemical Reaction Chemistrygod

Exothermic Stock Illustrations 60 Exothermic Stock Illustrations Vectors Clipart Dreamstime
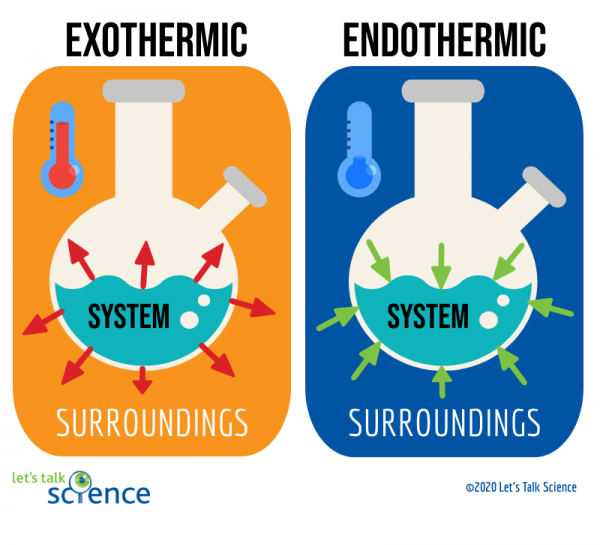
The Cold Pack A Chilly Example Of An Endothermic Reaction Let S Talk Science
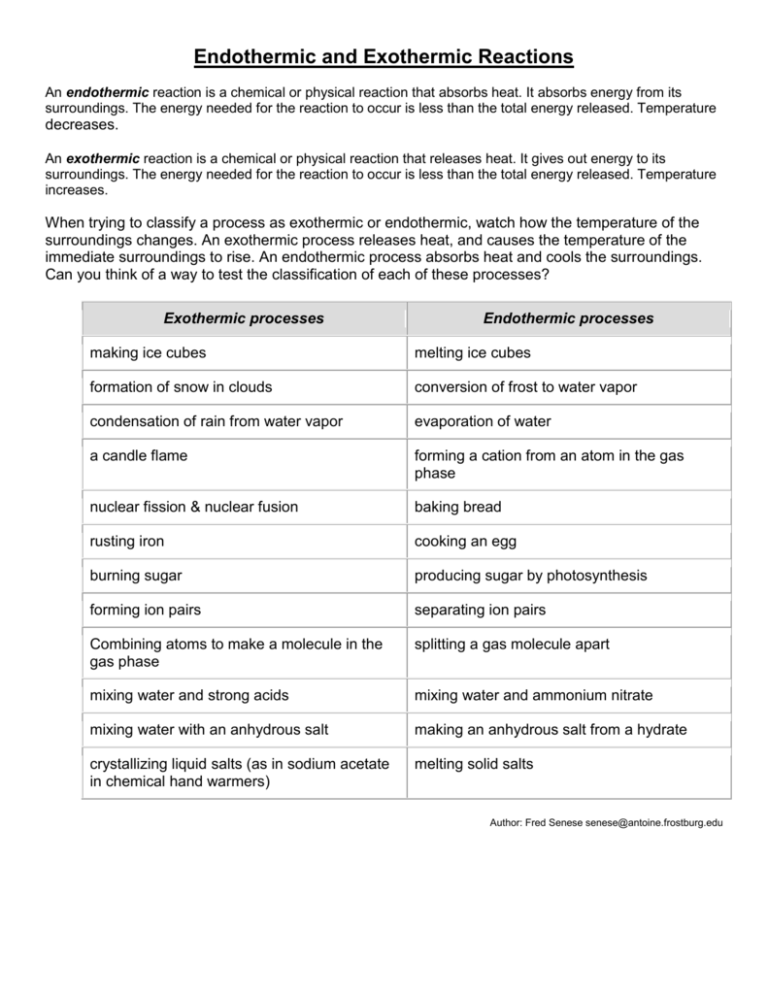
Endothermic And Exothermic Reactions

Unit 4 Endothermic Exothermic Flashcards Quizlet

Endothermic Exothermic Chemical Reactions Classifying Reactions Reactions Can Be Classified Based On Whether The Change Involves An Absorption Of Ppt Download

212 Exothermic Reaction Stock Photos Pictures Royalty Free Images Istock
Comments
Post a Comment